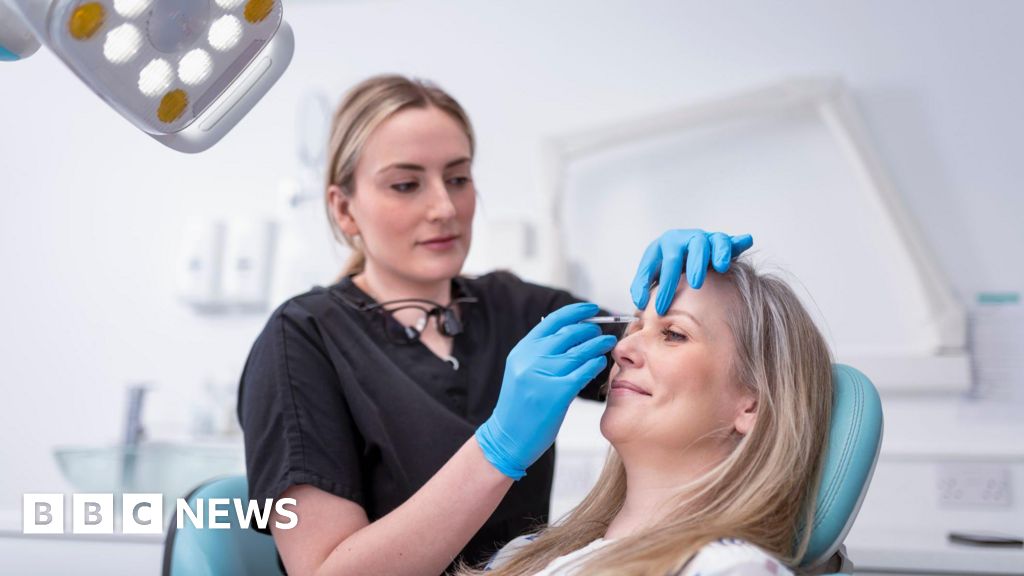
Thirty-eight cases of botulism poisoning have been recorded in England in the last six weeks after cosmetic procedures suspected to have involved the use of unlicensed Botox-like products, the UK Health Security Agency (UKHSA) said.
Botulism is a rare but life-threatening condition caused by toxins produced by Clostridium botulinum bacteria.
Cases have been recorded in the East, East Midlands and the North East regions.
The UKHSA urged those seeking treatments to obtain proof that their Botox practitioner was qualified and that their products were licensed.
Botox injections are a common cosmetic procedure given to reduce facial lines and wrinkles.
The product is made from small, purified doses of botulinum toxin, produced by the bacterium Clostridium botulinum. Larger doses can cause botulism.
According to the UKHSA, the evidence so far suggests clinics involved in the cases have used unlicensed Botox-like products.
In the most recent cases, recorded in East England and the East Midlands, patients had difficulty swallowing, slurred speech and breathing difficulties requiring respiratory support.
Other symptoms of botulism can include droopy eyelids, double vision and weak facial muscles.
Dr Gauri Godbole, of UKHSA, said botulism related to aesthetic procedures was rare but could be serious. She added that symptoms could take up to four weeks to develop and urged anyone who suspected they were suffering to contact the NHS 111 service.
Dr Alison Cave, chief safety officer at the Medicines & Healthcare products Regulatory Agency, said botulinum toxin was only available through prescriptions written by qualified healthcare workers.
“Buying botulinum toxin in any other circumstances significantly increases the risk of getting a product which is either falsified or not licensed for use in the UK.
“This means that there are no safeguards to ensure products meet the MHRA’s standards for quality and safety.”
The Joint Council for Cosmetic Practitioners says it receives numerous reports of the “illicit supply and use of unlicensed botulinum toxins”.
It suggests those considering Botox injections ask for information about the product, including its brand and intended dose, before accepting a procedure.
People should check these details again with the person carrying out the procedure on the day of their treatment. The prescription for Botox must be in the customer’s name.
The UKHSA recommends the following precautions:
- Make sure a practitioner is qualified, is wearing appropriate protective equipment and washes their hands. Practitioners should be happy to discuss their qualifications
- Those seeking a procedure should be offered a consultation beforehand that covers checks for medical conditions
- A consent form outlining the risks should be discussed and signed.