Victoria GillScience correspondent, BBC News
 Washington Post via Getty Images
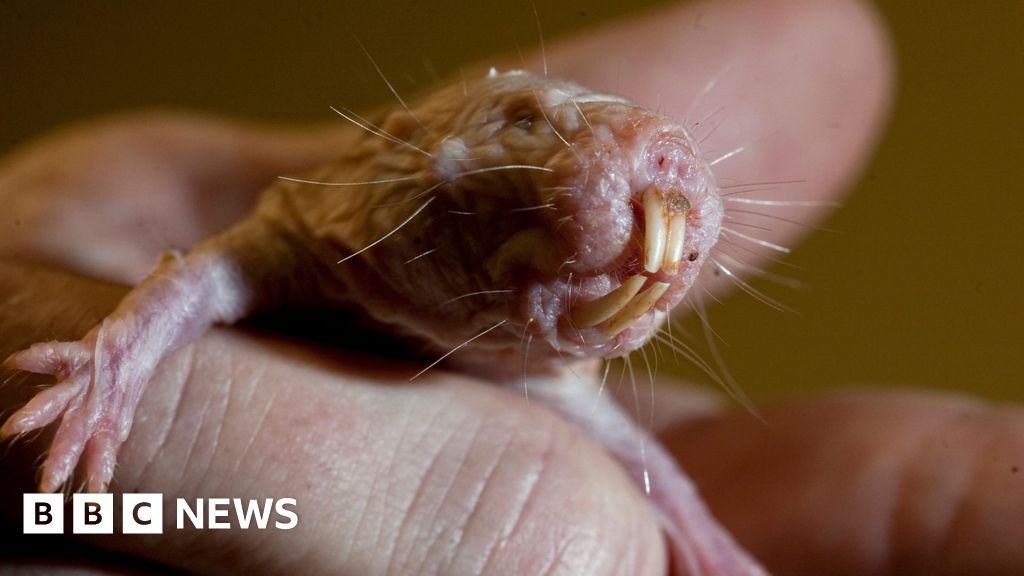
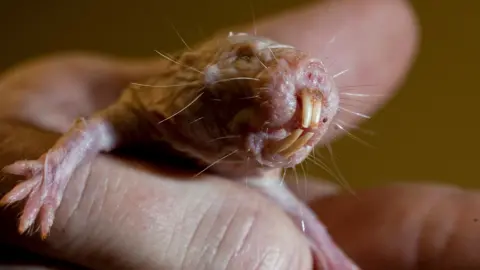
Washington Post via Getty ImagesThey are weird, bald, subterranean rodents that look like sausages with teeth, and they have just revealed a genetic secret to long life.
A new study of the bizarre naked mole rat shows that the animals have evolved a DNA repair mechanism that could explain their longevity.
These burrow-dwelling rats have a maximum life span of nearly 40 years, making them world’s the longest-lived rodent.
The new findings, published in the journal Science, could also shed light on why naked mole rats are resistant to a wide range of age-related diseases.
The animals are resistant to cancer, deterioration of the brain and spinal cord, and arthritis, so many scientists want to understand more about how their bodies work.
For this study, led by a team at Tonji University in Shanghai, China, the focus was DNA repair – a natural process in our bodies’ cells. When strands of DNA – our genetic building blocks – are damaged, a mechanism is triggered whereby another undamaged strand of DNA is used as a template to repair the break.
The focus of this research was on a particular protein that is involved in that system of damage sensing and repair.
When a cell senses the damage, one of the substances it produces is a protein called c-GAS. That plays several roles, but what was of interest to these scientists is that in humans, it interferes with and hampers the process by which DNA is knitted back together.
Scientists think that this interference could promote cancer and shorten our lifespan.
In naked mole rats though, the researchers found that the exact same protein does the opposite. It helps the body mend strands of DNA and keeps the genetic code in each cell intact.
 Chicago Tribune via Getty Images
Chicago Tribune via Getty ImagesProfessor Gabriel Balmus studies DNA repair and ageing at the University of Cambridge. He said the discovery was exciting and “the tip of the iceberg” when it comes to understanding why these animals live such extraordinarily long lives.
“You can think of cGAS as a biological Lego piece – the same basic shape in humans and naked mole-rats, but in the mole-rat version a few connectors are flipped, allowing it to assemble an entirely different structure and function.”
Over millions of years of evolution, Prof Balmus explained, naked mole-rats appear to have rewired the same pathway and “used it to their advantage”.
“This finding raises fundamental questions: how did evolution reprogram the same protein to act in reverse? What changed? And is this an isolated case or part of a broader evolutionary pattern?”
Most importantly, scientists want to know what they can learn from these rodents to improve human health and extend quality of life with age.
“I think if we could reverse-engineer the naked mole-rat’s biology,” said Prof Balmus, “we might bring some much-needed therapies for an ageing society.”